Trilogy EV300 and Trilogy EVO O2 Field Safety Notice
This is a Philips URGENT Field Safety Notice for the Trilogy EV300 and Trilogy Evo O2 that could pose a risk for patients or users. This letter is intended to inform you about:
- what the problems are and under what circumstances they can occur,
- the actions that should be taken by the customer/user in order to prevent risks for patients, and
- the actions planned by Philips to correct the problem.
Important Points from USME
- Devices can remain in use
- The software fix will come in a separate communication and field action
- In the EFS Calibration issue (issue 1), the device will alarm as designed. This issue has no reported customer complaints/incidents and was discovered during our follow testing on the Pressure Drift issue only.
- Pressure drift (issue 2) is associated with long-term use (2cmH2O over a month of uninterrupted use) High pressure alarms will annunciate when pressure threshold is reached.
1. What the problem is and under what circumstances it can occur
Two software issues have been identified related to pressure increase. The first issue is described as Infant/Pediatric EFS Calibration Pressure Increase and the second issue is described as Pressure Drift (Continuous Usage).
Issue 1 – Infant/Pediatric EFS Calibration Pressure Increase
An increase in the expiratory pressure (EPAP/PEEP) can occur when the pediatric/infant External Flow Sensor (EFS) is used with an Active Flow or Dual Limb circuit and a manual circuit calibration is performed. This increase in pressure will be seen shortly after starting therapy within approximately 1 to 2 minutes. The maximum expiratory pressure increase may reach up to 10 cmH2O above the set pressure. The inspiratory pressure (Pressure Support/Pressure Control/IPAP) may also be affected.
Issue 2 – Pressure Drift (Continuous Usage)
When a Trilogy EV300 or Trilogy Evo O2 model is used continuously without any interruption of therapy over weeks to months, the baseline pressure (that is, the pressure initially set for the patient) may increase or decrease at a rate of up to approximately 2 cmH2O per month. This increase applies to the PEEP and the inspiratory pressure at the same rate. The maximum pressure deviation that could be seen is a 10 cmH2O shift from the baseline pressure. The pressure regulation alarms will not annunciate with this issue.
This document contains important information for the continued safe and proper use of your equipment. Please review the following information with all members of your staff who need to be aware of the contents of this communication. It is important to understand the implications of this communication.
This issue applies to the following modes: CPAP, PSV, S/T, A/C-PC, SIMV-PC, SIMV-VC (PEEP and Pressure Support), and A/C-VC (PEEP). The User Interface (display screen) displays the actual pressure that the patient is receiving, which will differ from the baseline setting when this condition occurs.
2. Describe the hazard/harm associated with the issue
Issue 1 – Infant/Pediatric EFS Calibration Pressure Increase
There is potential for inappropriate therapy to be provided when the EFS is used with manual calibration. Harms that could occur as a result of an increase in delivered pressure include:
- Barotrauma (an injury to the lungs because of too much pressure)
- Hypotension (a decrease in blood pressure)
If the expiratory pressure (EPAP/PEEP) increases but the inspiratory pressure (Pressure
Support/Pressure Control/IPAP) remains unchanged, the following harm could occur:
- Hypercarbia (too much carbon dioxide in the blood)
Issue 2 – Pressure Drift (Continuos Usage)
There is potential for inappropriate therapy to be provided to a patient if there is a pressure increase or decrease from the baseline pressure settings without alarm. Harms that could occur as a result of pressure drift increase over time include:
- Barotrauma (an injury to the lungs as a result of too much pressure). The potential occurrence of barotrauma related to this issue is considered to unlikely; however, if it were to occur, it may be a serious injury.
- Hypotension (a decrease in blood pressure). The potential occurrence of hypotension related to this issue is considered to be occasional.
Harms that could occur as a result of pressure drift increase over time, and then abruptly reverting therapy (pressure settings) back to the initial prescribed settings, include:
- Dyspnea (a sensation of shortness of breath)
- Hypoxemia (a low amount of oxygen in the blood)
Harms that could occur as a result of pressure drift decrease over time include:
- Hypoxemia (a low amount of oxygen in the blood)
3. Affected products and how to identify them
Product name, Product number
Trilogy Evo O2: DE2100X13B, DS2100X11B, EE2100X15B, ES2100X15B,
EU2100X15B, FR2100X14B, FX2100X15B, IA2100X15B,
IN2100X15B, IN2100X19, IT2100X21B, JP2100X16B, LA2100X15B,
ND2100X15B, RDE2100X13B, SP2100X26B
Trilogy EV300 CA2200X12B, DS2200X11B, FX2200X15B, IN2200X15B
Trilogy EV300 and Trilogy Evo O2 devices with software versions 1.02.01.00, 1.03.05.00, 1.03.07.00,
1.04.02.00, 1.04.06, 1.05.01 and 1.06.02 are impacted.
To check the software version, tap the Options icon located on the top left side of the screen. Choose “Information” to find the software version.
4. Describe the actions that should be taken by the customer / user in order to prevent risks for
patients or users
Issue 1 – Infant/Pediatric EFS Calibration Pressure Increase
Please take the following actions until the upcoming software fix is implemented on your device.
If you are using the infant/pediatric EFS, do not perform a manual circuit calibration. Instead, use the
default calibration. The pressure increase will not occur when the default circuit calibration is used.
As stated in the Clinical Manual, the ventilator is optimized for circuits that are within the specifications
shown in “Circuit Requirements” section of the Clinical Manual, which are listed below. When using
the default circuit calibration settings, ensure that you are using a circuit with these specifications:
- Inspiratory/expiratory resistance: up to 5 cmH2O at:
o 15 L/min for pediatric (14 to 16 mm)
o 2.5 L/min for infant (9 to 13 mm) circuit size
- Compliance: up to 4 ml/cmH2O
If your circuit does not meet these criteria, then seek other options as stated in the Clinical Manual. When setting volumes that are greater than or equal to 50 ml, passive and active PAP circuit types may be used. If the above options are not clinically appropriate, then seek an alternative ventilator.
Issue 2 – Pressure Drift (Continuous Usage)
The following methods should be used to inspect the device to determine if a pressure drift has occurred as the pressure alarms will not detect it:
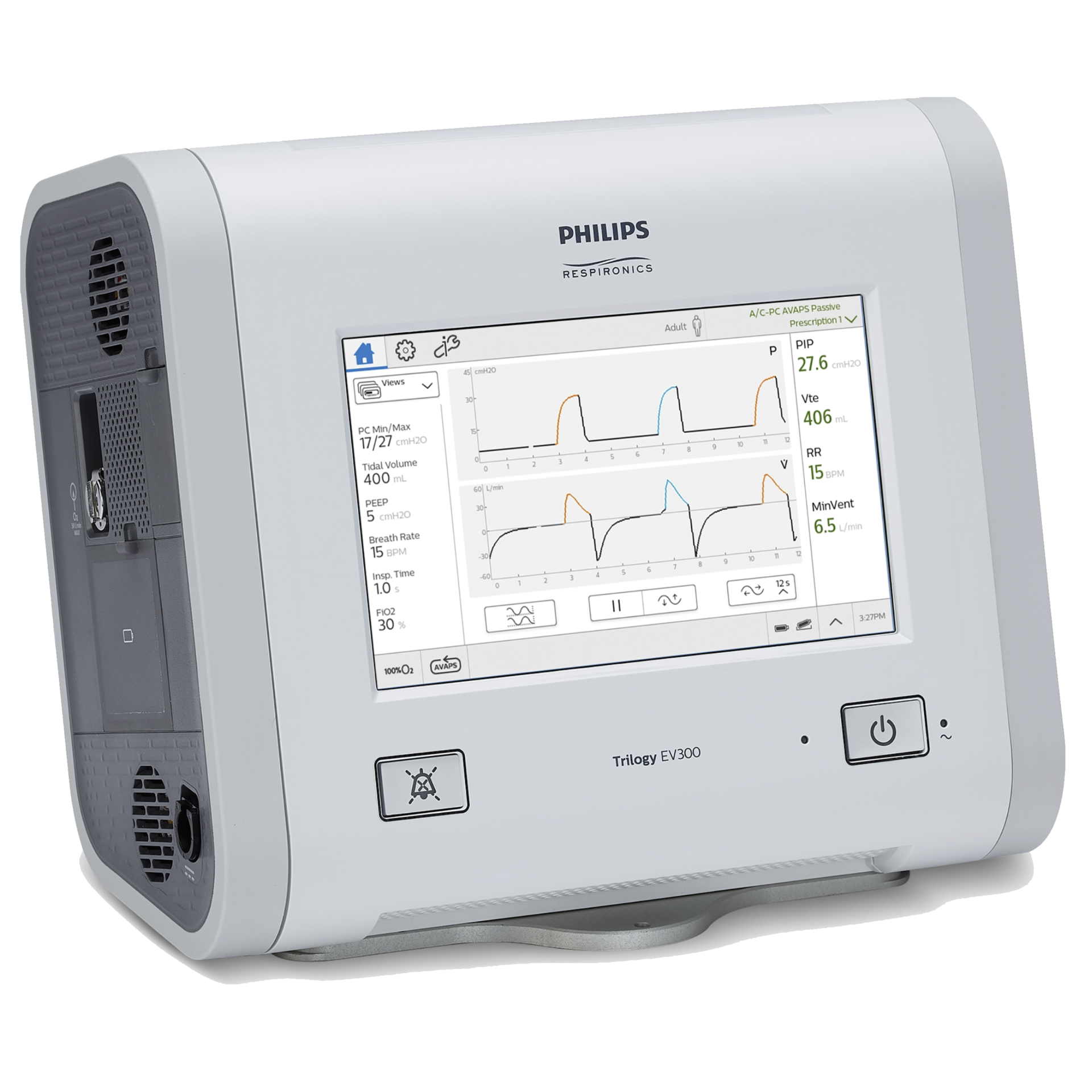
The caregiver/clinician can detect the increase in pressure by observing the measured parameters on the device’s screen (measured PIP value or pressure waveform as shown below where the IPAP is set to 15 cmH2O but because of the pressure increase the actual delivered pressure is 16.9 cmH2O).
If a pressure drift is detected, the decision to reset the device and return to its original, intended settings MUST be made in consultation with a clinician. Take steps to support the patient during a brief interruption in therapy, as needed.
Philips recommends that users periodically (monthly) perform the steps above to prevent the pressure from drifting.
5. Describe the actions planned by Philips SRC to correct the problem
Philips is releasing this Urgent Field Safety Notice to make customers and consignees aware of the potential issues described above and how to address them. Philips will be releasing a software correction for this issue. Philips will be contacting Trilogy EV300 customers when the software is released. If you need any further information or support concerning this issue, please contact your local Philips representative: <Philips representative contact details to be completed by the Market> Please complete, sign, and return the acknowledgment and receipt form at the end of this letter.
Should you have any questions, please reach out to the US Med-Equip Quality Assurance Team at QA@usmedequip.com.
Philips regrets any inconvenience caused by this problem.
About US Med-Equip
US Med-Equip (USME) partners with top hospitals across the nation to provide the highest quality movable medical equipment for patients in their care. USME, an Inc. 5000 Fastest Growing company, supplies quality-certified equipment rented, sold, maintained and managed using the latest technology to help healthcare providers focus on their patients’ healing. Contact USME today for your critical care equipment rental needs, medical equipment rentals, or medical equipment for sale.
